Abstract
Background: Frailty has been associated with increased rates of mortality in patients with malignancies. Studies have evaluated the role of frailty after hematopoietic cell transplantation (HCT) and found it to be associated with increased non-relapse mortality (NRM) and decreased overall survival (OS). Frailty has not been extensively studied among patients who develop chronic graft-versus-host disease (cGVHD).
Objectives: The objectives of this study were to assess the prevalence and predictors of frailty and the effects of frailty on transplant outcomes such as OS and NRM in patients enrolled in the Chronic GVHD Consortium. Patients were characterized as frail if they met Fried's definition: ≥3 of the following criteria at enrollment based on the 2 minute walk test and patient-reported outcomes: unintentional weight loss, exhaustion, slow walking speed, low physical activity, and weakness. Patients meeting <3 criteria were considered not frail. Chronic GVHD variables were measured at the time of enrollment. Because cGVHD severity is calculated from organ scores, models included cGVHD severity or organ manifestations, but not both. OS and NRM were calculated from the time of enrollment. Patients with newly diagnosed and established cGVHD were included; this was not associated with frailty or other outcomes, thus the two groups were combined for analysis.
Results: This study included 399 patients from 9 centers in the United States with 32.3% characterized as frail and 67.7% as not frail. At enrollment, cGVHD was severe in 29%, moderate in 54%, and mild/less than mild in 17%. The median follow-up time was 9.3 years.
Frailty was associated with higher severity of cGVHD (p<.001). In a multivariable analysis that excluded cGVHD severity, thrombocytopenia (OR 1.6, 95% CI 0.9-2.9, p=0.08), lung cGVHD (OR 2.4, 95% CI 1.5-4.0, p<0.001), liver cGVHD (OR 1.9, 95% CI 1.1-3.3, p=0.01), and older age (p=0.003) were associated with frailty.
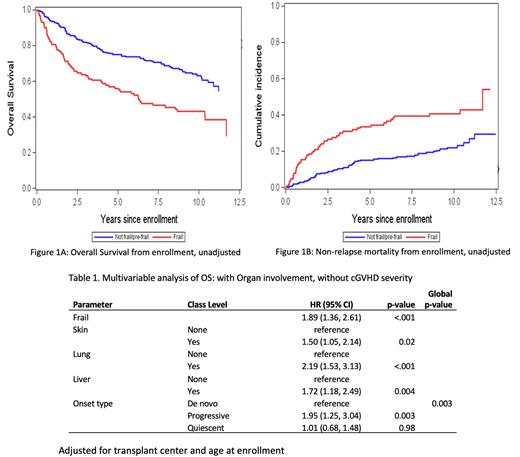
Frail patients had a significantly lower 10-year OS (Figure 1, p<0.001), 43% (95% CI 34-52%) vs 63% (95% CI 57-69%) and higher 10-year NRM (p<0.001), 41% (95% CI 32-49%) vs 22% (95% CI 17-28%) than not frail patients. In multivariable analysis, cGVHD severity was associated with NRM (p=.006) but not OS (p>.10) while frailty remained strongly associated with both endpoints (p<.001). There was no significant interaction between cGVHD severity and frailty in the NRM (p=0.44) or OS model (p=0.99). In a multivariable analysis (Table 1) excluding cGVHD severity and adjusting for older age (p=0.004) and transplant center (p=0.02), shorter OS was associated with being frail, having progressive-type onset of cGVHD, and having skin, lung or liver involvement. Results were similar for NRM (data not shown).
Conclusions: Among patients who have cGVHD, frailty remains strongly associated with worse OS and higher NRM after adjusting for cGVHD severity or organ manifestations. Patients older than 55 years and with lung or liver cGVHD are at higher risk of both being frail and for having worse transplant outcomes. Patients who develop cGVHD, especially in higher risk groups, may benefit from clinical and rehabilitation interventions to alleviate frailty.
Disclosures
Carpenter:Johnson & Johnson: Honoraria; Fate Therapeutics: Consultancy; Intellisphere: Consultancy; Pharmacyclics: Research Funding; Incyte: Research Funding. Alousi:Mallinkrodt: Honoraria; Prolacta: Consultancy; Sanofi / Kadmon: Honoraria; Incyte: Honoraria, Research Funding; Genetech: Consultancy. Hamilton:Equilium: Membership on an entity's Board of Directors or advisory committees; Syndax: Membership on an entity's Board of Directors or advisory committees; Nkarta: Membership on an entity's Board of Directors or advisory committees; Incyte: Membership on an entity's Board of Directors or advisory committees; Kadmon: Membership on an entity's Board of Directors or advisory committees. Pidala:Novartis: Research Funding; Syndax: Consultancy, Membership on an entity's Board of Directors or advisory committees; CTI Biopharma: Consultancy, Membership on an entity's Board of Directors or advisory committees; Amgen: Consultancy, Membership on an entity's Board of Directors or advisory committees; Regeneron: Consultancy, Membership on an entity's Board of Directors or advisory committees; Incyte: Consultancy, Membership on an entity's Board of Directors or advisory committees; Takeda: Research Funding; Johnson and Johnson: Research Funding; Pharmacyclcis: Research Funding; Abbvie: Research Funding; BMS: Research Funding. Cutler:Cimeio: Current equity holder in private company; CTI BioPharma: Consultancy; BMS: Consultancy; Equilium: Consultancy; Omeros: Consultancy; Mallinckrodt: Consultancy; Pfizer: Consultancy; Sanofi: Consultancy; Incyte: Consultancy; Jazz: Consultancy; Deciphera: Consultancy; Editas: Consultancy; CareDx: Consultancy; Janssen: Consultancy. Arai:Kadmon: Membership on an entity's Board of Directors or advisory committees. Lee:Kadmon: Consultancy, Honoraria, Research Funding; Pfizer: Research Funding; Equillium: Consultancy, Honoraria; Syndax: Research Funding; Mallinckrodt: Consultancy, Honoraria; AstraZeneca: Research Funding; Amgen: Research Funding; Incyte: Research Funding; Novartis: Other: Steering Committee; National Marrow Donor Program: Membership on an entity's Board of Directors or advisory committees.
Author notes
Asterisk with author names denotes non-ASH members.